Statistical Analysis
About Us
We develop analysis plans and carry out the analysis process based on statistical analysis expertise and extensive experience.
We plan the clinical trial design, set the number of cases, prepare the analysis plan, and analyze and evaluate the collected data.
We own the largest resources in Japan. We provide a variety of specialized services including CDISC standard support, intermediate analysis, implementation of multiple statistical methods, and pharmacokinetic analysis. We fully utilize our extensive experience to support regulatory submissions and reexamination applications from the statistical analysis perspective. Full-Support from statistical analysis planning to analysis results report. With a wealth of experience in clinical trials, post-marketing surveillance and statistical analysis of several clinical studies, we can handle a series of tasks, from protocol development support, creation of deliverables compliant with CDISC standards, implementation of pharmacokinetic analysis, and reporting of analysis results.EPS features
Services
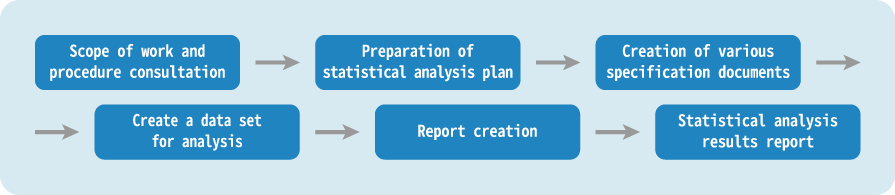
Main Services
