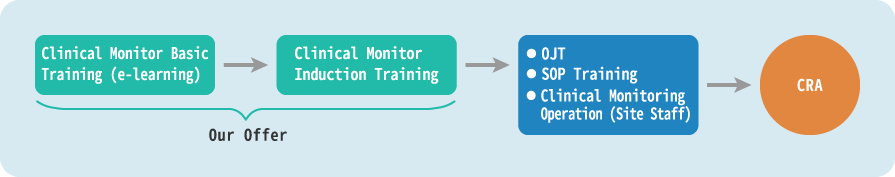
Clinical Monitor Training
About Us
We offer a practical and effective clinical monitoring training service that combines educational technology and classroom training.
We use our expertise in the CRO business to provide training support services for clinical monitors, including efficient and homogenous training through educational technology (e-learning) and practical classroom lectures given by dedicated teachers with extensive development experience. This saves you money and time and provides a practical and effective training service with proven high-quality.
EPS Features
Effective Training
We provide an effective training format that combines e-learning, classroom lectures and role-playing, allowing participants to acquire basic knowledge about clinical monitoring.
Learning Facilitation
Daily review tests and periodic confirmation tests promote knowledge retention.
Up-to-date Legal Compliance
Training will be provided in line with the latest legal changes.
Services
e-Learning
- In-house training attendance with ID and password management.
- Professional narrators provide easy to hear narrations.
- GCP comprehension test with 100 questions in addition to the lecture.
- Answers and explanations to test questions to increase comprehension.
Curriculum
- Basic Clinical Monitoring Training (first half)
- Code of Ethics in Drug Development
- GCP Ordinance
- Informed Consent
- Clinical Trial Review Committee
- Adverse Events and Basic Terminology Comprehension
- Health Damage Compensation
- Costs Associated with Clinical Trials
- Quality Control and Quality Assurance of Clinical Trials
- Basics of Data Management
- Clinical Trials Classification
- Clinical Trials Procedures
- Basic Clinical Monitoring Training (latter half)
- Drug Development Explanation
- Clinical Trial Implementation Overview
- Pharmaceutical Laws and Regulations
- International Regulatory Harmonization
- Post-Marketing Surveillance
- Clinical Trial Summary
- Non-Clinical Studies
- Role of the Clinical Trial Monitor
- Clinical Trial Summary Report
Clinical Monitor Induction Training
Monitors are required to understand the regulatory requirements (such as GCP), the high ethical standards and scientific knowledge as a clinical development professional, and the ability to negotiate with clinical trial researchers. The training to meet the forementioned requirements is provided by EPS for about four weeks to those who wish to become clinical monitors.
The teachers are veteran monitors currently active in the clinical field, providing practical lectures based on their experience.
The training is not only in the form of lectures to learn knowledge, role-playing training is also provided to simulate direct communication with principal investigators and other clinical trial personnel.
Remote Classroom Lecture
- Clinical Monitoring Introduction
- Clinical Trials Laws and Regulations
- Clinical Trials Protocol Structure and Contents
- Clinical Trials Review Committee
- Clinical Trials Cost
- Data management
- Clinical Monitoring Operation (from patient selection to termination procedure)
- Pharmaceuticals and Ethics
- Pharmacovigilance
- Informed Consent Forms
- Compensation and Indemnity
- CRC (Clinical Research Coordinator) Operation
- Statistical Analysis
- FDA`s ALCOA, etc.
Role-playing (remote and direct)
- Selection
- Request Procedure
- Briefing Session
- SDV (Source Data Verification)
