GCP Audit
About Us
We evaluate and verify clinical trials from an independent standpoint.
Auditing is a part of quality assurance that evaluates and verifies whether clinical trials and clinical research are being conducted in compliance with relevant laws, regulations, guidelines, protocols, standard operating procedures, etc.
We play a role in improving the credibility of clinical trials and clinical research by evaluating and verifying the results independently from monitoring or quality control operations.
EPS Features
High Precision
Our staff, who are familiar with laws, regulations, and guidelines related to clinical trials as well as the concept of ISO 9001, will appropriately evaluate and verify compliance with various requirements.
High Efficiency
We provide efficient and high-quality audits based on our extensive business experience.
Wide Coverage Area
From Sponsor-Initiated Clinical Trials to PI-Initiated Clinical Trials, we audit a wide range of clinical trials and clinical research.
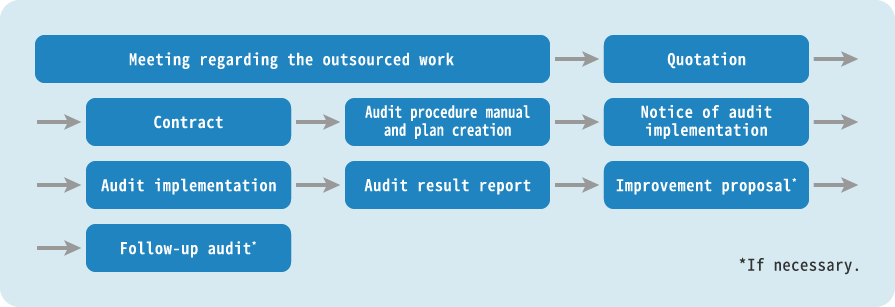
Services
- Clinical trials and clinical research for pharmaceuticals, medical devices, and regenerative medical products audit.
- Medical institutions audit.
- Audit for Internal or external organizations involved in the implementation of clinical trials.
- Audits for Sponsor-Initiated Clinical Trials to PI-Initiated Clinical Trials.
We perform a variety of audits related to clinical trials and clinical research