Finding from Services
New Initiatives, Opening Doors to Clinical Trials, Proposals for Clinical Trial Reforms, Post-Manufacturing and Sales, PV Models

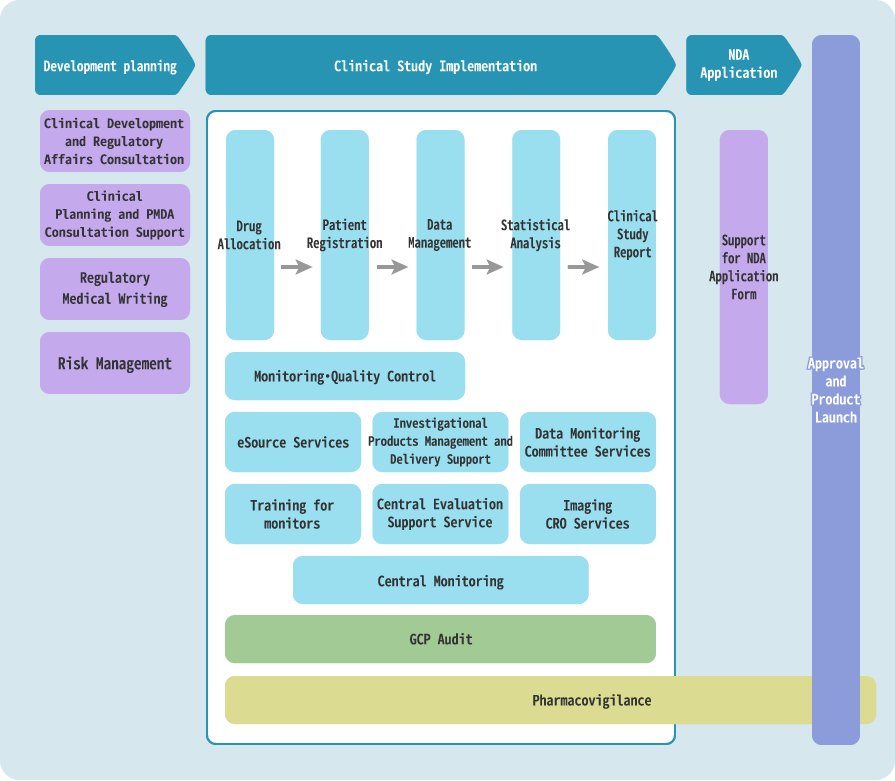
Clinical Trials
We provide full support for quickly developing high-quality pharmaceuticals and regenerative medical products.
EPS handles all CRO operations in clinical trials.
For multiple outsourcing services, our project managers specialized in clinical development provide strong support as an integrated contact point for the entire outsourcing service, making full use of their expertise to meet our client’s needs.
Leveraging EPS Group economies of scale, we contribute to the speedy and high-quality development of pharmaceuticals and regenerative medical products by supporting clinical trials in Asia and other parts of the world, and by providing clinical development solutions with even higher added value.
We provide services in the medical device development area in collaboration with our group company EP Mediate Co., Ltd.
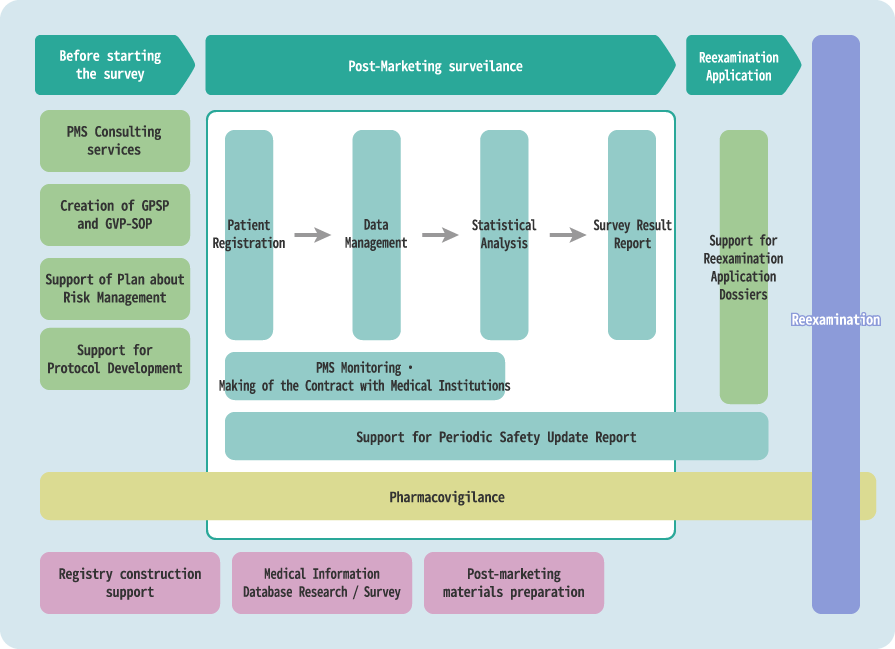
Post-Marketing Services
Based on our expertise and abundant experience, we provide a one-stop service for quick, high-quality post-marketing services.
We support all Post-Marketing CRO operations.
When required to handle all post-marketing operations, we promise to provide you with even greater benefits through smooth coordination between services.