Patient Registration
About Us
Accurate and speedy eligibility determination and subject assignment in compliance with protocols.
Patient Registration is the process of including subjects (patients) in a clinical trial.
The Patient Registration Center provides a mechanism for investigators and sub-investigators to obtain the information necessary to determine the eligibility of potential subjects through randomization and trial supply management (RTSM), fax or telephone, and to properly incorporate them as subjects when their eligibility is confirmed.
When using RTSM, eligibility is based on the information entered on the Internet. When using telephone or fax, eligibility determination and subject assignment are conducted based on the information obtained and entered by an operator.
EPS Features
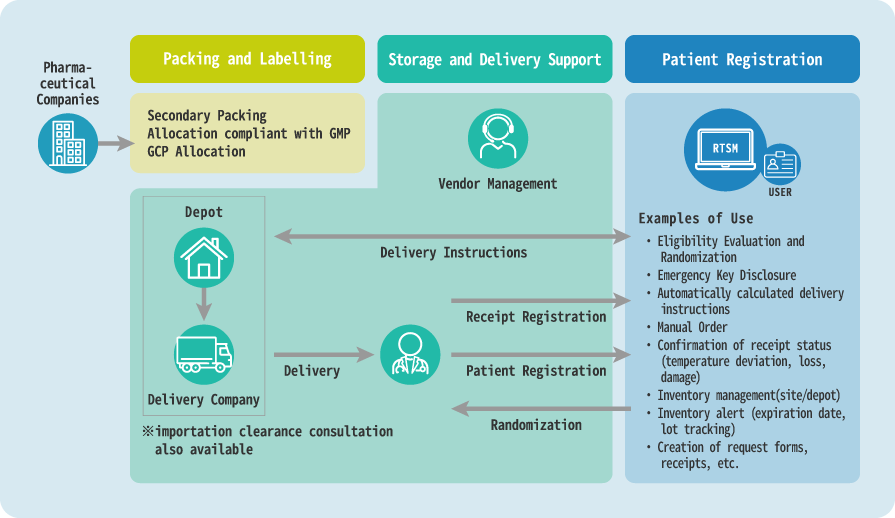
One-Stop Services (investigational drugs packing and labelling, drug allocation, storage and delivery management, patient registration and randomization).
Project Management
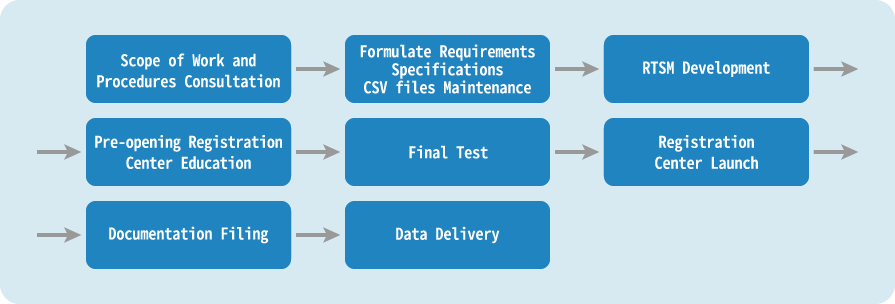
In addition to preparing patient registration operation procedures that comply with GCP (Good Clinical Practice), adequate education and training is provided to operators in accordance with quality control processes.
Accuracy
Static allocation (stratified allocation, permuted blocks, etc.) based on key codes and dynamic allocation using the minimization method is also available. With dynamic allocation, it is possible to carry out a simulation in advance based on the planned allocation method, check the performance, and implement it in the registration system.
High Efficiency
Regarding investigational drug delivery instructions, we propose the most appropriate algorithm and implement solid supply management according to the client's most important points and study design, such as manufacturing cost, delivery cost, and personnel expenses.
Services
Speedy Qualification Check and Reliable Supply Management under Strict Quality Control
We open a patient registration center based on the needs of the clinical trial to register and allocate subjects.
We accept subject entries through a central registration system using RTSM, telephone, and fax, and follow strict quality control standards to obtain credible data that can withstand analysis.
Main Services
- RTSM specification design and system development
-
[Patient Registration]
- Eligibility criteria
- Allocation of treatment groups in randomized controlled trials (dynamic allocation and static allocation, etc.)
- Allocation of drugs used and administration groups
- Dose calculation (titration) for each visit
- Emergency key disclosure
-
[Investigational Drug Management (medical devices and regenerative medical products)]
- Automatic calculation of the number of drugs to be delivered and delivery instructions / manual delivery instructions
- Investigational drug warehouse and site inventory management
- Drug collection and disposal status management
-
[Others]
- Data linkage with external systems (inspection data, EDC, etc.)
- Various types of reporting
- We offer optimal systems in conformance to different requirements such as study design and budget.
- We also offer propositions that uses the RTSM of EDC.
-
[Patient Registration]
- Patient Registration Center Services
- RTSM Helpdesk for site and user information management
- Acceptance of patient registration via Web, FAX, and TEL
- English-speaking operators
- Help desk for not only RTSM but various systems (EDC, ePRO, etc.) for site and user information management
- Emergency call reception service(Available 24 hours a day, 365 days a year)
