QualityLead (Sample/Reagent Management,Compound libraries)
What is QualityLead?
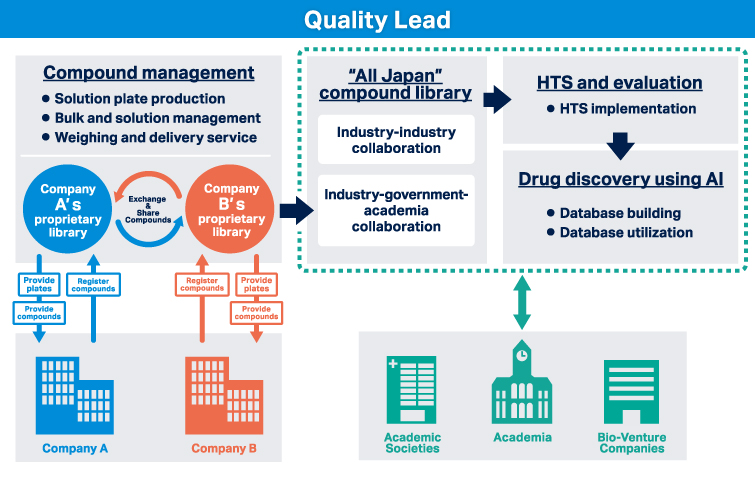
This is a service that consolidates and manages compound libraries (compounds for drug discovery research) owned and stored individually by pharmaceutical companies in facilities and platforms provided by us. It is expected that this service will have the following effects.
- Reduction of investment and fixed costs at the drug discovery stage by outsourcing the work of owning and managing compound libraries
- Increased productivity in drug discovery research and development
- Sharing of compound libraries among pharmaceutical companies
- BCP measures by distributing compounds to our facilities
QualityLead Benefits
Reducing the workload of pharmaceutical companies
- There is no need for pharmaceutical companies to individually manage compounds
- Significant reduction in capital investment and fixed costs to manage compounds
Creation of opportunities for academia
- Academia can easily access corporate libraries
- Increasing opportunities to match the drug discovery seeds of academia with the needs of pharmaceutical companies to create new drugs
Realization of AI Drug Discovery
- Building a database of shared compounds
- Drug discovery researchers can widely utilize the constructed database for data mining
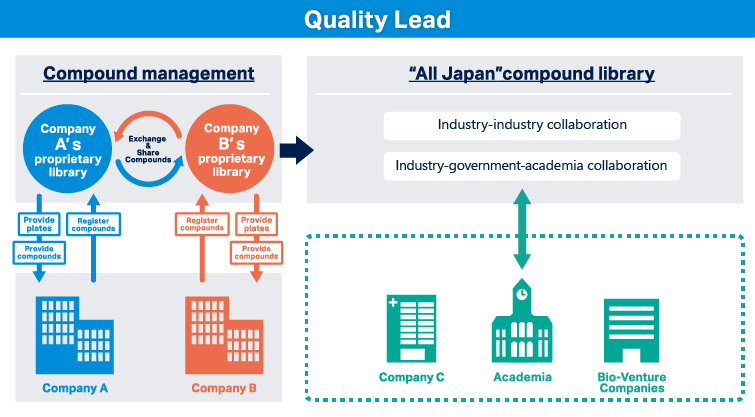
Operation of “All Japan” Compound Library
- Providing a platform for shared library operation in industry-industry and industry-academia collaboration
Main Contract Services
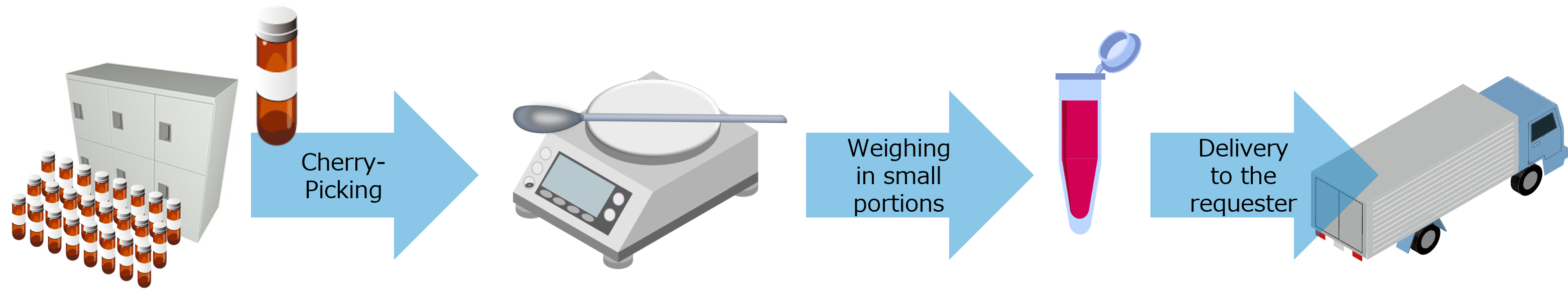
Storage management of Bulk
- Initial transfer and storage management of library compounds stored at pharmaceutical companies (bulk powder and vials) to QL Service.
- Transfer of new library compounds (bulk powder and vials) to QL Service after initial transfer and their storage management.
- Weighing of transferred compounds upon request
- Provide from the bulk storage upon request.
- Management of all compounds in the vault, including residual quantities and disposal, is managed in a database and reported as inventory information at any time.
- Disposal of library compounds (bulk powder) in accordance with revisions of laws and regulations upon request.
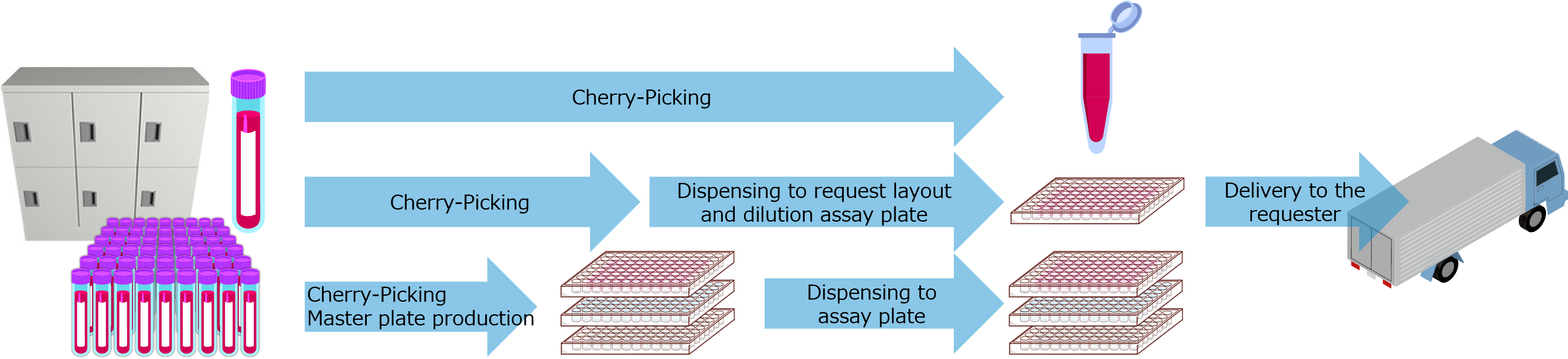
Management of solution storage and plate production
- Initial transfer of 96 tubes and 384 tubes of compound solutions stored at pharmaceutical companies
- Transfer of new library compounds (solutions, tubes or plates) to QL Service after initial transfer
- Plate production by shipping out individual tubes from the 384-tube automated storage
- Production of cherry-picked plates of hit compounds from the primary screening (for reproducibility testing)
- On-demand library plate production for hit compounds and other compound searches
- Production of assay ready plates from storage plates
- Provide compound solutions from the solution storage upon request
- Management of all compound solutions in the vault, including residual quantities and disposal, is managed in a database and reported as inventory information at any time.
Available as a platform for shared library participation
Providing a platform to use compound libraries deposited in the QL Service as shared libraries among clients
- Shared library: Group 1
- A platform for participating companies to share and use shared compounds provided by compound solutions handled by QL Service.
- To participate, 50,000 or 100,000 compounds must be submitted.
- Shared library: Group 2
- A platform where shared compounds provided from compound solutions handled by the QL service are shared with consortium participating companies via a certain secretary company of the consortium.
- To participate, 1,000 to several tens of thousands of compounds must be submitted.
- Shared library: Group 3
- A platform for sharing relatively small-scale shared compounds provided by compound solutions that are handled through the QL service among participating companies.
- To participate, 5,000 or 10,000 compounds must be submitted.
Support for collaborative research (primary compound management and plate production services for collaborative research, etc.)
- Entering customer-supplied plates into storage as source plates
- Production of assay ready plates from source plates
- Support for production of assay ready plates and picking plates for a certain period of time
Business Continuity Plan (BCP) support
- QualityLead service manages compounds as a countermeasure against disasters such as earthquake, flooding, and fire in the customer's compound storage facility.
