Risk Management Plan Creation
About Us
Consistent safety measures support, from drug development to post-marketing
We propose a scientific and efficient risk management plan in compliance with the latest regulations and handle the entire process, including the preparation of the draft version of the Risk Management Plan (RMP) for submission.
EPS Features
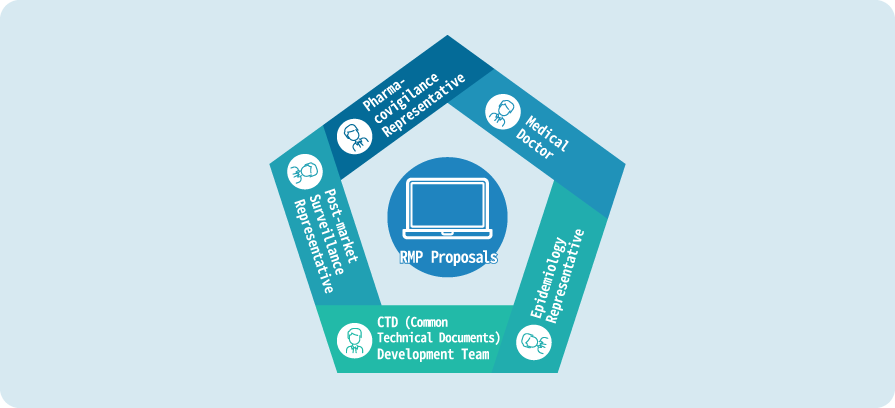
Team Structure
- We have a dedicated review team with a wealth of experience from clinical trials to post-marketing.
- Necessary examinations and proposals will be made by a cross-departmental examination team consisting of specialized staff with various backgrounds in pharmaceuticals.
- The team includes pharmacovigilance officers, medical doctors, epidemiologists, CTD (Common Technical Documents) development team, post-marketing surveillance officers, and instructional developers.
Flexibility
- We make proposals focused on the response of the authorities.
- We provide flexible and detailed support and proposals based on pre-application consultation with the authorities.
Services
- Creation of RMP proposals for submission.
- Preparation of materials to be discussed with overseas RMPs.
- Preparation of risk assessment materials based on clinical trial data of the product and safety data derived from similar products.
- After establishing the risk proposal, review and discuss the concerns, consider the research question (RQ), and prepare the records.
- Review and propose a post-market surveillance plan by framing a RQ to the PICOT format
- Review and propose a risk-minimalized plan based on relevant issues.
- Propose risk term grouping definition
- RMP periodic review
Other Services
- Translation (Japanese to English and English to Japanese)
- Quality Control
