Pharmacovigilance
About Us
Experienced and professional staff provide prompt and accurate support.
In Pharmacovigilance response operations, safety information collected from various sources such as clinical trials, post-marketing surveillances and studies, voluntary reports, literature conference, and adverse event reports from overseas is entered into a database, primary processing (primary evaluation) is performed, and if necessary, a re-investigation proposal to medical institutions and a report proposal to the PMDA are prepared.
In addition, for clinical trials and post-marketing clinical trials, we prepare and deliver proposal documents to be submitted to the principal investigator and the head of the medical institution.
EPS Feature
Full Support
Experienced staff with proficiency in English provide full support and assistance for both domestic and overseas operations.
Adverse Events
We provide consultation with medical specialists within EPS and contracted external medical specialists for adverse events that are difficult to detect.
High Efficiency
We collaborate with EPS Group's data management, statistical analysis, medical writing, and clinical development departments to efficiently support our customers' operations.
Services
Experienced and Professional Staff Available
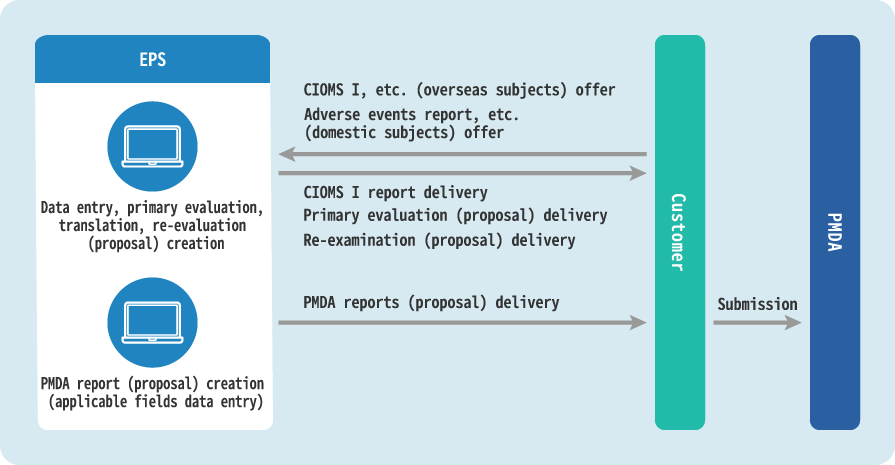
Main Services
- Primary evaluation and Japanese translation of overseas information using CIOMS I and MedWatch forms.
- Input information from clinical trials held in Japan and post-marketing Pharmacovigilance into the Safety Database.
- Primary screening and primary evaluation of literature conference information.
- English translation (CIOMS I and MedWatch creation).
- Instructional support for monitors and medical representatives.
- Preparation of various report proposals for clinical trials and post marketing Pharmacovigilance (side effects and infectious disease case report, research reports, measure reports, clinical trial device malfunction and adverse event case report, etc.).
- Clinical trials and post-marketing Pharmacovigilance report proposal creation (unknown and non-serious periodic reports, safety portion of periodic safety reports and reexamination application documents, annual clinical trial safety reports, PBRER, PSUR, DSUR, RMP, etc.).
- Infectious diseases periodic report primary evaluation and report proposal creation.
- Consulting on overall Pharmacovigilance management operations and other related operations.
- Adverse event consultations with EPS`s contracted specialist physicians.
- Preparation of various reports (internal safety evaluation committee documents proposal, overseas headquarters and partner companies report proposals).
