Central Monitoring
About Us
A group of experts in risk management and data analysis provide Central monitoring tailored to the study characteristics.
Our Central monitoring supports not only risk management activities, but also evaluates trend and outliers in each indicator (KRI, etc.) across sites to confirm the risk status of studies and select the on-site monitoring process.
EPS features
Proactive Involvement in Relevant Departments
To maximize the effectiveness of Risk Management and Risk-Based Monitoring (RBM) in clinical trials, it is essential for Central monitoring to be actively involved in the relevant departments. Central monitoring not only facilitates but also provides suggestions and support to maximize the performance of the concerned departments.
Staff with Specialized Knowledge
Our Central monitoring team is composed of people with clinical knowledge, QMS and Risk Management expertise, and the ability to evaluate and explain results appropriately, as well as people who are familiar with EDC structure/external data and have knowledge in building analytical tools and CSV to support your operations.
Support for a Variety of Systems and Commissioning Formats
From system development to Central monitoring and risk management, we provide a wide range of services. We support various systems such as BI tools (Spotfire, Tableau), RBM system (CluePoints), Medidata Solutions' Targeted SDV, CSA (Centralized Statistical Analytics), and others.
Services
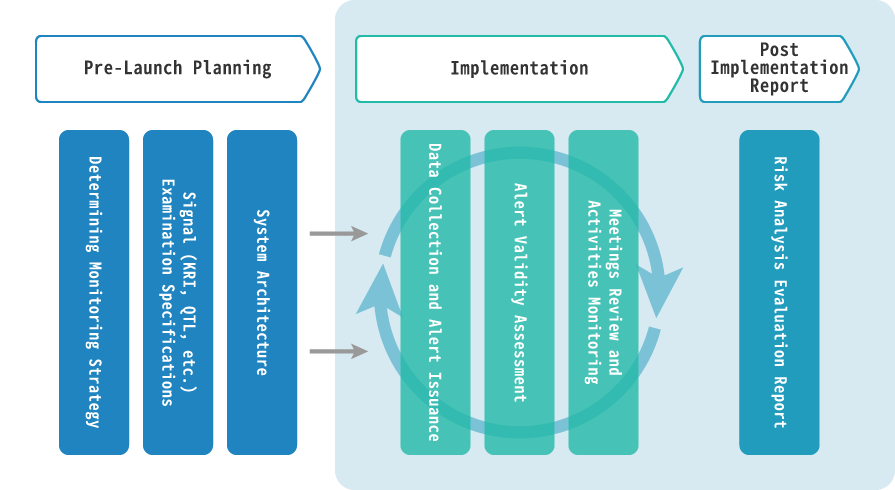
Pre-Launch Planning
- Central Monitoring Preparation
- Determine specifications of signals (KRIs, QTLs and statistical Central Monitoring, etc.) to be used as risk reviews.
- Central monitoring protocol creation.
- System Architecture
- System specifications settings.
- System validation.
- Creation of data to be imported into the system.
Implementation
- Central Monitoring
- Analysis and evaluation of signals displayed in the system and alert reviews.
- Organize and operate review meetings between Central monitors and site monitors.
- Action instructions for monitoring activities.
- Quantitative validation of site monitors CAPA content compliance.
- System Operation Service
- System maintenance and inspection
Post-implementation Report
- Central monitoring results report creation.
Incidental Related Services
- Monitoring support materials (BI tools) creation.
Cloud version BI Tool, Tableau Online Building/Operation Services.
