Data Management eSource Services
About Us
We provide eSource Services that are required for Virtual Clinical Trials and Decentralized Clinical Trials (DCT).
We also offer one-stop service from preparation to completion of clinical trials, and hybrid services by combining conventional methods.
eSource (Electronic Source): Electronic records as the source materials
eSource Data: Original data recorded electronically
Data originally recorded electronically, and any information recorded in the original record or a certified copy of the original record regarding clinical findings, observations, or other activities necessary to reproduce and evaluate the trial.
Introduction Advantages
The ability to collect and check clinical trial data in real time.
Stated in the FDA guidelines, eSource is recommended by TransCelerate Biopharma Inc.
Ensure reliability, quality and traceability of clinical trial data.
Patient`s safety assurance.
Reduction of SDV man-hours.
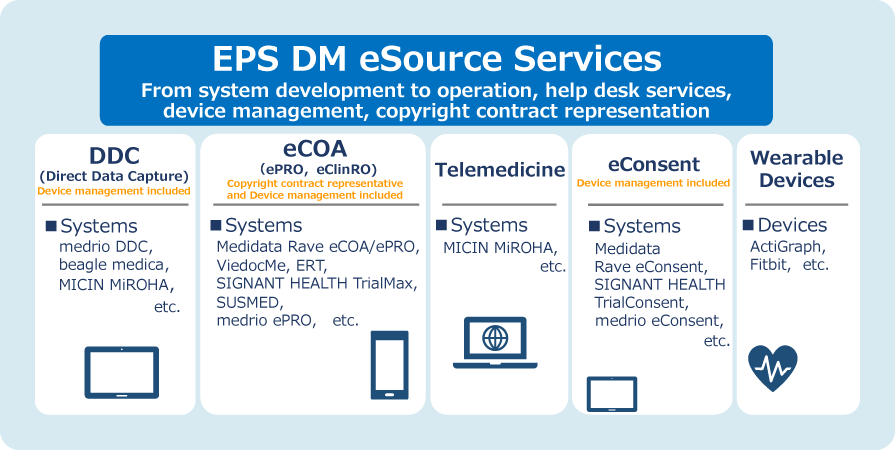
Services
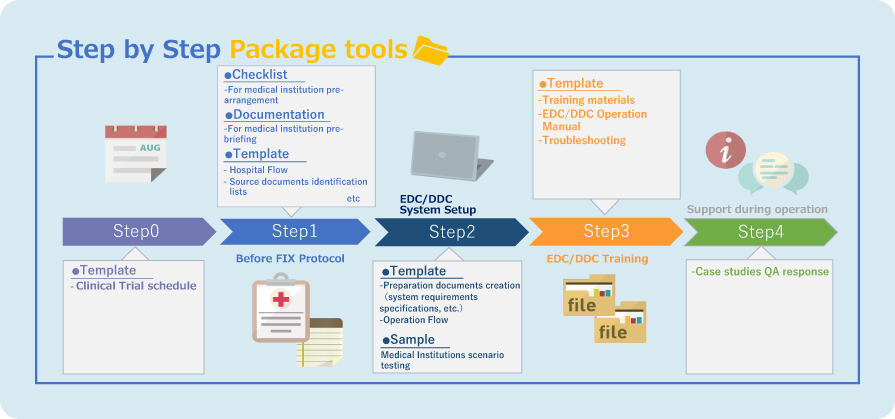
eSource (DDC) Standard Model
Reflect stakeholders' perspectives and cover necessary tasks for each step.
Smooth implementation even for first-time users.
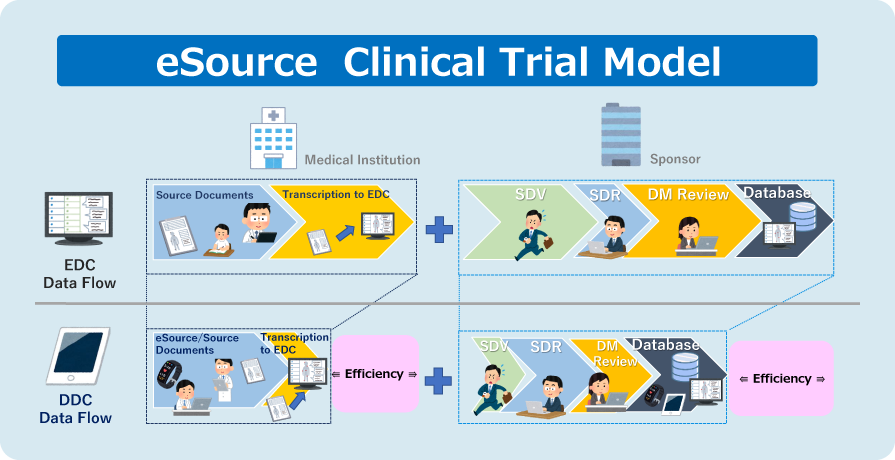
With the eSource flow, clinical data is collected immediately and efficiently by electronically capturing original documents as eSource data.
Even with only some parts of the existing data (patient diaries, worksheets, etc.) being converted to eSource data, efficiency improvement can be expected.
The use of validated systems helps improve credibility, quality, and immediacy.