Data Monitoring Committee Services
About Us
We provide detailed support, from committee experts arrangements to committee management.
The Data Monitoring Committee (DMC) consists of members with pertinent expertise that reviews clinical trials that evaluates interim data neutrally and objectively in ongoing clinical trials.
We provide appropriate advice and recommendations to the sponsor to ensure the safety of trial subjects and the continuing validity and scientific merit of the trials.
EPS' DMC services are based on the "Guidelines for Data Monitoring Committees" in Japan.
EPS Features
Experience
Being one of the top CROs in Japan we manage studies in a wide range of fields and phases.
By Region
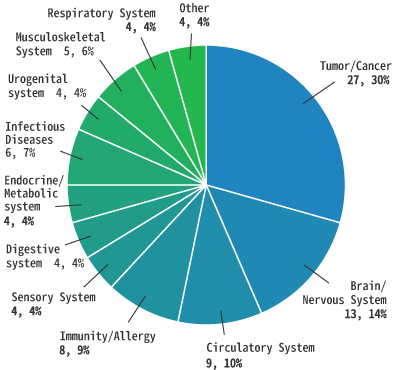
By Phase
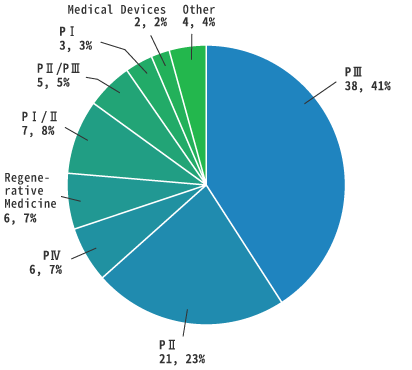
As of June 2021
Total: 92
Number of cases, percentage (%))
Expertise
Based on our past achievements, we can introduce medical and biostatistics specialists in various fields as DMC members. We also provide a wide range of services to first-in-human studies for medical devices and regenerative medical products and deliberations of an adaptive clinical trial design.
When conducting interim analysis or aggregation, we collaborate with the person in charge of the interim analysis and proceed with deliberations while adding explanations from a statistical perspective.
In addition to the concepts of independence, objectivity, and blinding, a dedicated team trained in project management and communication plans will be in charge of the DMC, and will work with the necessary parties to ensure that the information firewall is functioning properly.
Online services
The procedure for holding open and closed deliberations using the web conference system has been standardized and has been used in many cases.
Services
We provide total support for necessary operations from DMC establishment to its management.
Main services
- DMC procedure manuals creation
- Selection of DMC committee members, side job application and contract support
- Conflicts of interest management
- Kick-off meetings
- Provision of up-to-date materials to DMC members
- Data review support for pharmacovigilance (periodically and urgently)
- Venue and transportation arrangement
- Web conference preparation
- Preparation, distribution, collection, and disposal of conference materials
- Conference moderation
- Minutes and recommendations creation
- Sealing and storage of confidential discussion documents
- DMC committee members' work expenses payment support
