Clinical Trial Monitoring
About Us
EPS aims towards a partnership that meets the demands for quality, speed, productivity, expertise, and availability in the face of today`s changing needs.
Clinical Trial Monitoring is the process of ensuring that the human rights and safety of subjects are protected and that clinical trials are conducted, recorded, and reported in compliance with the latest study protocols and Good Clinical Practice (GCP) guidelines.
EPS’ Strengths
Quality
Our Clinical Research Associates (CRAs), who have achieved a high level of specialized knowledge and communication skills through specialized training, accurately conduct monitoring operations for pharmaceuticals, medical devices, and regenerative medicine products in accordance with the latest PMDA and GCP regulations.
In addition, we have implemented cQMS* for all our studies, and by operating a quality management system that utilizes EPS's extensive knowledge, we can guarantee high quality services and deliverables.
※cQMS:clinical Quality Management System
Speed
Delays in subject enrollment is the main cause of clinical trial delays, which end up setting back the availability of new drugs and increase development costs.
Through information obtained from our extensive experience in past trials and collaboration with EP-Link, one of the largest Site Management Organizations (SMO) in Japan, we conduct highly reliable feasibility investigation and smooth subject recruitment.
Productivity
With changes in the clinical trial environment, such as the increasing difficulty of development and the complexity of study plans, improving the productivity of clinical trial monitoring is an urgent issue.
As partners, we propose a flexible resource plan according to the difficulty of the study and schedule, and more efficient monitoring methods (central Monitoring, cooperation with EP-Link, remote monitoring, virtual studies, etc.).
Expertise
We have an excellent track record in the field of oncology, CNS, and regenerative medicine, which require a high level of expertise and are highly evaluated by pharmaceutical companies and medical institutions conducting clinical trials.
With knowledge to cover all types of cancer, the oncology area accounts for most active trials monitored by EPS.
Based on our proficiency, we confidently propose study operations that lead to successful clinical trials.
Supply Capability
With 1,000 CRAs, the largest number in Japan, we are able to shorten development lead times and respond flexibly to sudden changes in development strategy. In addition, with CRAs experienced in the field of Oncology, CNS, and regenerative medicine products, we can allocate staff to specialized areas.
Services
Full Support for Monitoring from Phase I to Phase IV
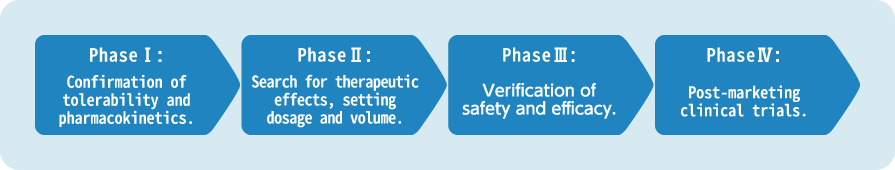
While consenting to client`s requests, our CRAs, who have undergone specialized training, carry out the work in each phase in compliance with various procedures manuals as well as GCP guidelines.
Main Services
- Creation of various procedure manuals.
- Feasibility studies.
- Site selection.
- Request and contract procedures.
- Start-up meetings.
- Compliance with relevant regulations and protocols, and process management verification
- Source Data Verification (SDV).
- Collection and confirmation of case report forms.
- Monitoring reports preparation.
- Collection and provision of safety information.
Incidental Services
- Preparation of various study materials (Informed Consent drafted by the sponsor (for domestic use only), etc.).
- Local study manager in Japan for global and domestic studies.
- Preparation of site contracts.
- Disbursement services
Quality Management
Ensuring quality that meets various regulatory requirements from an objective perspective.
Our Quality Control (QC) specialists ensure quality by objectively verifying whether clinical trials are being conducted in compliance with GCP guidelines, related laws, and other regulations and whether the processes are adequate.
We also handle the storage and management of medical devices, including specially controlled medical devices.
Document Management
Ensuring quality by managing study-related documents according to the client`s needs.
We handle both paper and electronic test-related documents to meet your requirements.
To ensure quality, we manage obtained and provided test-related documents in customized storage facilities.
Electronic documents will be properly managed in the customer's eTMF system or an eTMF system provided by EPS.
Human Resource Development
Ensure quality that meets various regulatory requirements from an objective perspective.
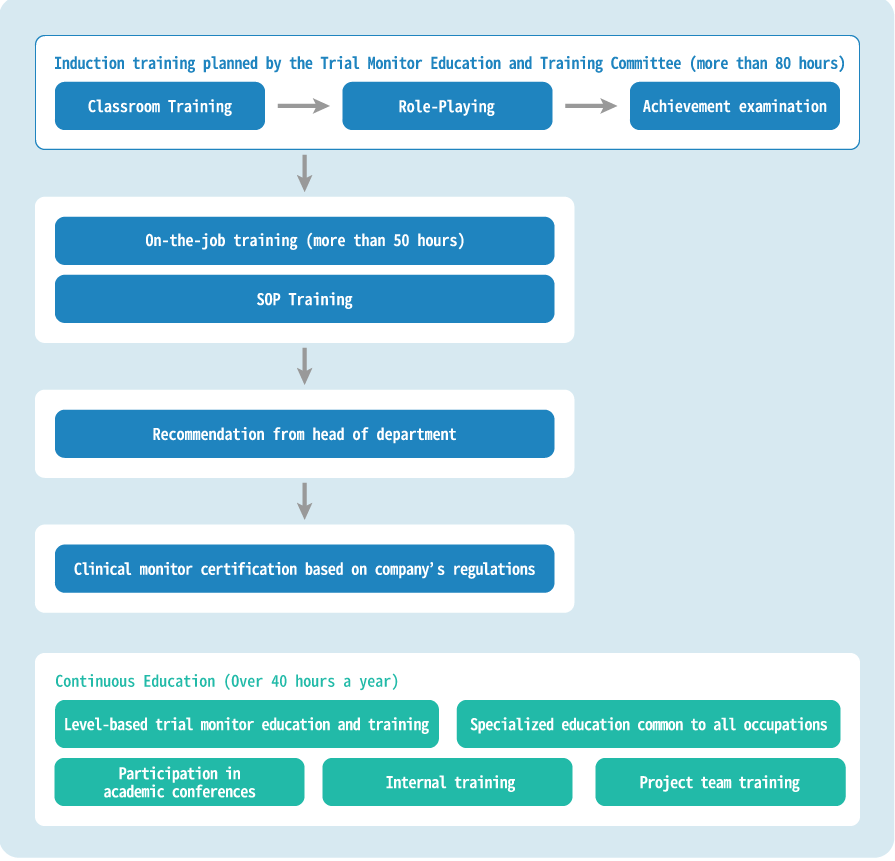
We have established a Monitor Education and Training Committee, a dedicated organization for human resource development, which plans and implements training programs to enhance professional knowledge and improve operational capabilities.
We provide classroom training and role-playing in the introductory education, conduct examinations to check achievement levels, and certify CRAs through on-the-job training. In addition to these training programs, mentors are assigned to inexperienced CRAs to provide further practical and specialized continuous education, training CRAs to be immediately effective.
Furthermore, based on our extensive experience in Oncology, we have a variety of Oncology training programs (e-Learning and Oncology Academy).